Proper food labeling is critical for dietary supplements and nutrition products to meet FDA guidelines and provide clear, accurate information to consumers. Whether you’re labeling packaged food or similar products, adhering to labeling requirements ensures compliance and helps inform informed choices.
This food label guide covers essential regulations for allergens, dosage, and ingredients, ensuring your product’s nutrition information is accurate and supports food safety. Our article provides a good overview of these regulations, helping you understand what you must fulfill to meet the FDA standards that will provide the transparency needed for certain foods in the market.
Allergen Labeling Requirements for Dietary Supplements
Accurate food labeling for allergens is essential in dietary supplements to ensure food safety and comply with FDA regulations. Food manufacturers must clearly identify allergens on labels to protect consumers with food allergies.
FDA’s Food Allergen Labeling and Consumer Protection Act (FALCPA)
The FDA’s Food Allergen Labeling and Consumer Protection Act (FALCPA) only applies to packaged foods intended for human consumption, including dietary supplements. It requires that any packaged food containing one or more of the nine major allergens (milk, eggs, fish, shellfish, tree nuts, peanuts, wheat, soybeans, and sesame) be clearly labeled. This law helps consumers make informed choices and avoid allergens that could affect their health. Products must list these allergens in both the ingredients list and a separate ‘Contains’ statement immediately following the ingredients list.
How to Identify Allergens on the Label
To comply with FDA rules, allergens must be easy to spot on the nutrition facts label or the ingredients list. Including clear allergen warnings helps consumers compare food products and avoid harmful ingredients.
‘Contains’ Statement
One effective method of allergen labeling is using a “Contains” statement. This statement must be placed immediately following or adjacent to the ingredients list on food packaging. It highlights any of the nine major allergens present, such as “Contains: milk, eggs, and wheat,” ensuring transparency for consumers and helping them make safe choices.
Cross-Contamination Warnings
In addition to the “Contains” statement, some products may include voluntary warnings about possible cross-contamination. Phrases like “May contain” or “Produced in a facility that processes” are often used to alert consumers that the product might have come into contact with major allergens. When packaged food is processed in shared facilities, these warnings are crucial for food safety.
Labeling for Less Common Allergens
Certain products may contain less common allergens in addition to the nine major ones. Food manufacturers must clearly disclose any other allergens found in their products or ingredients that could affect consumers. For example, some supplements may contain additional allergens beyond the nine major ones, such as mustard or lupin, which should be clearly stated on the food label to prevent allergic reactions.

Dosage Labeling Requirements for Supplements
Properly labeling dosage information is crucial to ensuring food safety and guiding consumers on using dietary supplements correctly, as outlined by FDA regulations and the Dietary Supplement Health and Education Act (DSHEA). Clear dosage instructions help users understand serving sizes and safe intake limits, promoting the safe consumption of your food products.
Displaying Correct Serving Size and Dosage Information
Accurate serving size information is essential on a nutrition facts label. It helps consumers understand how much of a supplement they are consuming. You should clearly list the serving size and the number of servings per container on the food label to ensure the user knows exactly how much to take. This is especially important for products where serving size affects the percent daily value of certain nutrients.
Dosage Instructions and Usage Directions
Displaying dosage instructions ensures that consumers use the product safely. This includes specifying how many servings to take, how often, and under what conditions. For example, you might recommend taking the product “with food” or “before bed” to enhance effectiveness or avoid side effects. These labeling requirements provide clarity to help users make informed choices.
Upper Limits and Safety Warnings
It is critical to provide guidelines on the maximum safe daily intake of the supplement, especially for ingredients with established upper limits. Including a warning about the risk of exceeding the recommended dosage ensures that consumers use the product responsibly. For example, nutrients like vitamins or minerals should list the percent daily value and any potential risks of overconsumption.
Special Population Warnings
Certain supplements may pose risks to specific groups of consumers, such as pregnant women, children, or people with medical conditions. It’s important to include clear warnings on the nutrition labels for these populations. This helps protect vulnerable groups and ensures compliance with FDA standards for food safety.

Ingredient Labeling Requirements for Supplements
Accurate ingredient labeling is essential for dietary supplements to comply with FDA regulations and provide consumers with clear, informative food labels. Proper labeling ensures transparency and safety, especially for supplements with complex formulations.
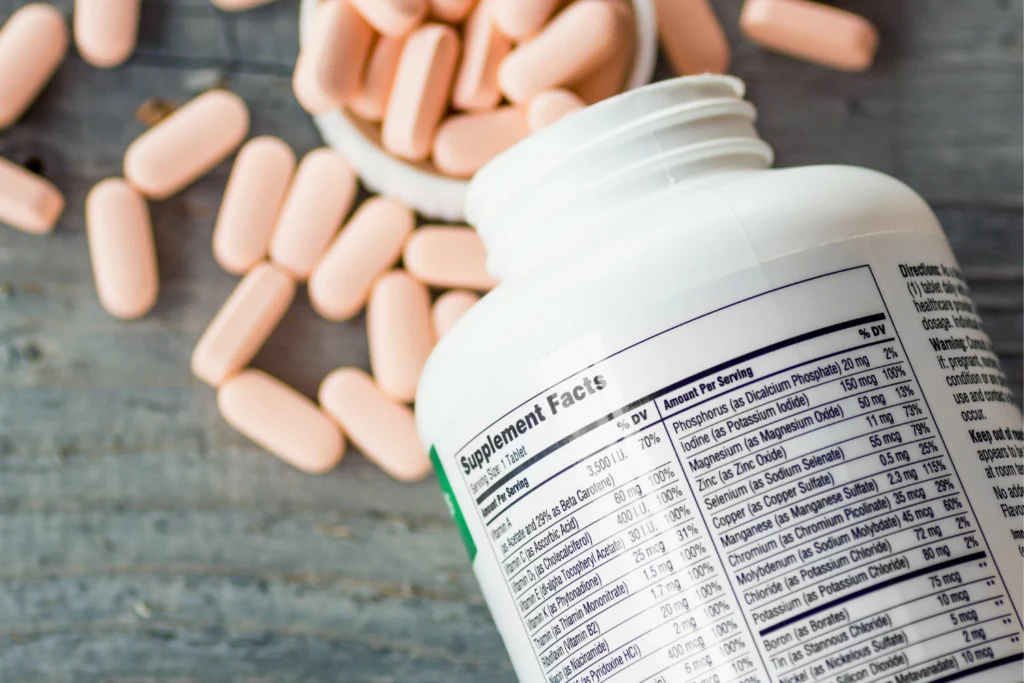
Supplement Facts Panel
The Supplement Facts panel provides a detailed overview of the product’s ingredients and nutritional content. This section must include essential information to help consumers make informed choices about their intake. Key components of the Supplement Facts panel are:
- Name of each dietary ingredient in the supplement
- Amount of each ingredient per serving
- Percent Daily Value (if applicable) for each nutrient
- Total servings per container
- Clear separation of added sugars, vitamins, and minerals
How to Display Active vs. Inactive Ingredients
It’s important to distinguish between active ingredients, which provide the desired health benefits, and inactive ingredients, like fillers or preservatives. For clarity, both types must be listed on the Supplement Facts panel. Here’s how to display them:
- List active ingredients separately from inactive ingredients
- Provide the specific amounts of each active ingredient
- Include a description of each inactive ingredient’s function (e.g., filler, binder)
- Use clear headings to differentiate the two types of ingredients
- Ensure consumers can quickly identify the health-promoting ingredients versus those that serve other purposes
Listing Ingredients in Descending Order
To comply with FDA regulations, food manufacturers must list ingredients in descending order based on their predominance by weight. This means that the ingredient making up the largest portion of the product comes first on the ingredients list, followed by smaller amounts. This helps consumers understand the concentration of each ingredient in the product.
Using Common or Scientific Names
For some nutrients or ingredients, it’s important to use both common and scientific names to avoid confusion. For example, consumers may recognize vitamin C, which may also be listed as ascorbic acid in the Supplement Facts panel. While not required, including both names helps improve clarity for consumers. Using both names helps clarify what’s in the product and provides consistency across food products.
Proprietary Blends and Transparency
When listing proprietary blends, manufacturers are allowed to combine several ingredients under one name. However, FDA rules require listing the total weight of the blend while listing the individual components in descending order by weight—without specifying the exact amounts of each ingredient within the blend. This gives consumers insight into the product’s content while maintaining the blend’s proprietary nature.
Emphasizing Transparency for Consumer Trust
Full transparency in ingredient labeling is key to building consumer trust. By clearly listing all ingredients and their amounts, you can set your product apart from similar products on the market. Consumers value honesty in labeling, and this practice can enhance your brand’s reputation and strengthen your position in a competitive market.
Additional Labeling Considerations for Compliance
Beyond allergens, dosage, and ingredients, dietary supplements must meet other labeling requirements. These ensure your product remains compliant with FDA regulations and provide full transparency to consumers.
Listing Fillers, Binders, and Preservatives
In addition to active ingredients, manufacturers must list all non-active components of the supplement. This includes fillers, binders, and preservatives that may be used in the product. These ingredients must be listed on the nutrition facts panel or ingredients list to fully inform consumers about what is in their supplements. Proper disclosure promotes food safety and helps consumers make informed choices about the supplements they use.
Labeling for Organic, Non-GMO, or Other Certifications
If your product carries third-party certifications like USDA Organic or Non-GMO Project Verified, it’s essential to display these seals correctly. Certification seals communicate important product attributes to consumers. Here are some guidelines:
- Display the certification seals clearly on the principal display panel
- Ensure the seals do not overshadow required nutritional facts
- Use official logos provided by the certifying body, such as USDA, for organic food products
- Ensure any claims, like “organic” or “non-GMO,” are accurate and meet the certification standards
- Include additional certifications, such as for animal welfare or sustainability, where applicable

Labeling Requirements for International Markets
Special labeling requirements apply when selling food products in international markets. Labels must be translated into the language(s) of the destination country, and you must comply with local regulations for allergens and ingredients. For instance, the nutrition facts panel and ingredients list should meet the specific guidelines of the target market, including the proper listing of allergens and local nutritional information standards.
Meeting FDA Labeling Requirements for Supplements
Ensuring your product’s food labels comply with FDA labeling requirements for allergens, dosage, and ingredients is essential for regulatory compliance and consumer safety. By following guidelines for allergen disclosure, listing proper dosage instructions, and providing a complete ingredients list, you enhance transparency and build consumer trust.
Accurate food labeling helps your product stand out while avoiding legal complications. If you’re unsure about compliance, seeking expert guidance ensures your labels meet all necessary FDA standards and helps maintain food safety.
Frequently Asked Questions
What allergens must I list on my supplement label?
You must list the nine major allergens: milk, eggs, fish, shellfish, tree nuts, peanuts, wheat, sesame and soybeans.
How do I ensure my dosage instructions meet FDA guidelines?
Display the serving size, recommended dosage, and specific usage conditions to meet FDA guidelines.
Do I need to list all inactive ingredients on my product label?
Yes, you must include all inactive ingredients like fillers, binders, and preservatives in the ingredient list.
What should I include on the Supplement Facts panel?
The Supplement Facts panel should list dietary ingredients, their amounts, and the % Daily Value, if applicable.
How do I label my product for international markets?
Translate your food label and ensure compliance with local allergen and ingredient labeling laws in each market.
References
- U.S. Food and Drug Administration. (2023). Allergic to sesame? Food labels now must list sesame as an allergen. U.S. Department of Health and Human Services. https://www.fda.gov/consumers/consumer-updates/allergic-sesame-food-labels-now-must-list-sesame-allergen
- U.S. Food and Drug Administration. (2022). Food Allergen Labeling and Consumer Protection Act of 2004 (FALCPA). U.S. Department of Health and Human Services. https://www.fda.gov/food/food-allergensgluten-free-guidance-documents-regulatory-information/food-allergen-labeling-and-consumer-protection-act-2004-falcpa
- U.S. Food and Drug Administration. (2024). Food allergies: What you need to know. U.S. Department of Health and Human Services. https://www.fda.gov/food/buy-store-serve-safe-food/food-allergies-what-you-need-know
- U.S. Food and Drug Administration. (2022). Guidance for Industry: Questions and Answers Regarding Food Allergen Labeling (Edition 5). https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-questions-and-answers-regarding-food-allergen-labeling-edition-5
- U.S. Food and Drug Administration. (2024). Label Claims for Conventional Foods and Dietary Supplements. https://www.fda.gov/food/food-labeling-nutrition/label-claims-conventional-foods-and-dietary-supplements
- U.S. Department of Agriculture. (n.d.). Labeling Organic Products. https://www.ams.usda.gov/rules-regulations/organic/labeling
- U.S. Department of Agriculture. (n.d.). Labeling Policies. https://www.fsis.usda.gov/inspection/compliance-guidance/labeling/labeling-policies
- U.S. Food and Drug Administration. (2005). Dietary Supplement Labeling Guide. https://www.fda.gov/food/dietary-supplements-guidance-documents-regulatory-information/dietary-supplement-labeling-guide
- U.S. Food and Drug Administration. (2013). Food Labeling Guide. https://www.fda.gov/files/food/published/Food-Labeling-Guide-%28PDF%29.pdf
- U.S. Food and Drug Administration. (2018). Guidance for Industry: Food Labeling Guide. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-food-labeling-guide



