Creating an accurate and compliant supplement facts label is crucial for dietary supplement manufacturers. A well-designed label ensures your dietary supplement products meet FDA regulations and helps consumers make informed decisions about what they’re putting into their bodies. From listing dietary ingredients to understanding daily values and nutrient content claims, proper supplement labeling is critical in building trust with customers and marketing claims.
Once you follow best practices and legal requirements, supplement manufacturers can ensure their labels provide the necessary information about serving size, total fat, and more, all while highlighting the health benefits of the product.

What is a Supplement Facts Label?
The FDA requires a supplement facts label for all dietary supplements to ensure transparency and compliance. This label provides critical information about the product’s dietary ingredients, serving sizes, and nutrient content, allowing consumers to make informed choices. This label is not just a formality; it’s a legal requirement that applies to all supplement products sold in the U.S.
These labels also help build trust between supplement manufacturers and customers by clearly showing what is inside the bottle. A well-structured supplement facts panel ensures that consumers know exactly what they are consuming, whether it’s vitamins, minerals, or other dietary ingredients. This transparency fosters confidence in your brand and helps customers feel secure about their health choices.
Regulatory Requirements for Supplement Facts Labels
The Food and Drug Administration (FDA) has strict regulations for supplement facts labels. These rules are essential to ensure that dietary supplement products meet legal standards and are safe for consumers. Dietary supplement manufacturers must include specific details such as the names of dietary ingredients, their amounts, and the recommended daily intake values. These FDA regulations are vital in ensuring transparency and preventing misleading claims.
By complying with FDA regulations, your supplement business can avoid costly legal issues and protect itself from penalties. An accurate and well-organized supplement facts label helps meet regulatory requirements and reassures your customers that your products are trustworthy and safe to use.
Key Elements of a Supplement Facts Label
A supplement facts label must contain specific details about your product. This includes information about serving size, nutrient quantities, ingredient lists, and daily values, all essential for compliance and consumer understanding.
Serving Size and Servings Per Container
The serving size must reflect how much of the supplement is recommended per dose. To calculate this, you should determine the amount of each active ingredient that provides the desired health benefits. The serving size must be clearly displayed on the supplement facts panel, making it easy for consumers to know how much of the product they should take.
Number of Servings Per Container
The number of servings per container refers to the number of servings included in the entire bottle or package. This is crucial because it helps customers understand the total amount of product they are buying and how long it will last based on the recommended serving size.

Nutrient Quantities and Daily Values
Your supplement facts label must list all key nutrients in your dietary supplement, such as vitamins, minerals, and amino acids. This section gives consumers detailed information about the amount of each nutrient and its contribution to their daily nutritional needs.
- Vitamin C content
- Total fat and saturated fat amounts
- Dietary fiber and total carbohydrates
- Amounts of calcium, iron, and potassium
- Any specific nutrient, such as amino acids or proprietary blends
Percent Daily Value (%DV)
The Percent Daily Value (%DV) shows how much a nutrient in a serving of the supplement contributes to a consumer’s daily diet. This value is based on FDA guidelines, which use a 2,000-calorie diet as a reference. Listing the %DV helps consumers easily understand if a product contains a high or low amount of a particular nutrient, allowing them to make informed health decisions.

Ingredient List
The ingredient list provides a breakdown of all the components in your supplement, including both active and inactive ingredients. Active ingredients are those that provide health benefits, like vitamins or amino acids, while inactive ingredients may include fillers or flavorings that do not directly affect health but are part of the product formulation.
Order of Ingredients
The ingredients in your supplement must be listed in descending order by weight. This means that the ingredient present in the largest amount is listed first, and the one in the smallest amount is listed last. Properly formatting the ingredient list helps ensure transparency and compliance with FDA regulations.
Other Required Information
The supplement facts label must also include the precise amount of each active nutrient or ingredient per serving. This is critical for consumers who want to monitor their nutrient intake closely. Additionally, the label should specify if any non-dietary ingredients, such as additives or preservatives, are used in the formula.
Additional Nutritional Information
Depending on the product, you may need to include extra details such as the total calories, fats (including saturated and trans fat), protein, and total sugars per serving. This additional nutritional information helps consumers evaluate the product based on their dietary goals, such as reducing sugar intake or increasing protein consumption.
Common Mistakes to Avoid on Supplement Labels
Even small errors in supplement labeling can lead to confusion or regulatory issues. Here are some of the most common mistakes to watch out for.
Inaccurate Serving Sizes
Incorrect serving sizes can mislead customers about how much of the product they should take. If the serving size listed is inaccurate, it can cause dosing problems and may result in customers not receiving the intended health benefits. Moreover, inaccurate serving sizes can lead to regulatory problems, as the FDA requires that the serving size be clearly and correctly displayed on the supplement facts label.
Missing Key Ingredients
Omitting important active ingredients from your supplement label can have serious consequences, including legal issues and losing customer trust. Every active ingredient in your dietary supplement must be listed.
- Leads to customer confusion
- Reduces transparency, affecting trust in your brand
- Results in non-compliance with FDA regulations
- May lead to consumer health risks
- Could cause legal penalties for misleading labeling
Forgetting Excipients or Fillers
Inactive ingredients, like fillers, binders, and preservatives (excipients), must also be listed on your supplement label. Even though they don’t provide health benefits, these non-dietary ingredients are crucial for product formulation and must be disclosed. Forgetting to include them could result in compliance issues with FDA regulations.
Incorrect Percent Daily Values
Miscalculating the percent daily values (%DV) of critical nutrients, such as vitamins and minerals, can mislead consumers about how much of a nutrient they’re getting. To avoid this, carefully follow FDA guidelines when calculating %DV for micronutrients and macronutrients, ensuring your label is accurate and trustworthy.
FDA Compliance: What You Need to Know
Meeting FDA guidelines for supplement labels is essential to avoid penalties and ensure your product is safe for consumers.
Label Layout and Design Requirements
The FDA provides specific guidelines for the layout and design of supplement facts labels to ensure readability. This includes requirements for font size, style, and the spacing of text. The correct format helps consumers easily read important details like serving size, nutrient quantities, and daily values. The label must be clear, easy to follow, and presented in a way that is consistent with FDA regulations.
Mandatory Placement of Supplement Facts Label
To comply with FDA regulations, the supplement facts label must be placed in a specific location on the product packaging. It should be placed in a panel format on the outer container or packaging, ensuring it is easily visible to consumers. This placement is crucial for legal compliance and helps customers access the necessary information quickly.
Common FDA Labeling Violations
Certain mistakes on supplement labels can lead to FDA violations, which can be costly for your business. Here are some common errors to avoid:
- Missing ingredient lists or omitting key components
- Inaccurate serving sizes or nutrient amounts
- Using unclear or misleading nutrient content claims
Making Unsubstantiated Health Claims
It’s important not to make health claims on your label unless backed by scientific evidence. Claims about specific health benefits, such as curing a disease or drastically improving health, must be substantiated. Unverified health claims can result in serious legal issues and damage your brand’s reputation. Always stick to claims that the FDA allows and are based on the actual effects derived from the ingredients in your supplement.
Best Practices for Designing Your Supplement Label
Designing a supplement label requires balancing eye-catching branding with FDA compliance. Here are the best practices to help you achieve that.
Branding and Compliance
When designing your supplement label, creating a visually appealing brand image is essential while staying compliant with FDA regulations. You can showcase your brand’s personality using colors, fonts, and logos, but the critical information—such as the supplement facts panel, serving sizes, and ingredients—must still follow legal guidelines. Ensure all required elements are correctly displayed without overcrowding the label, keeping the design attractive and compliant.
Highlighting Key Information for Consumers
Making critical information easy to find on your supplement label is vital for transparency and customer trust.
- Place the serving size and servings per container at the top of the supplement facts label.
- Clearly display each nutrient’s % Daily Value (%DV) to help consumers make informed choices.
- Ensure that active and inactive ingredients are in a prominent, easy-to-read location.
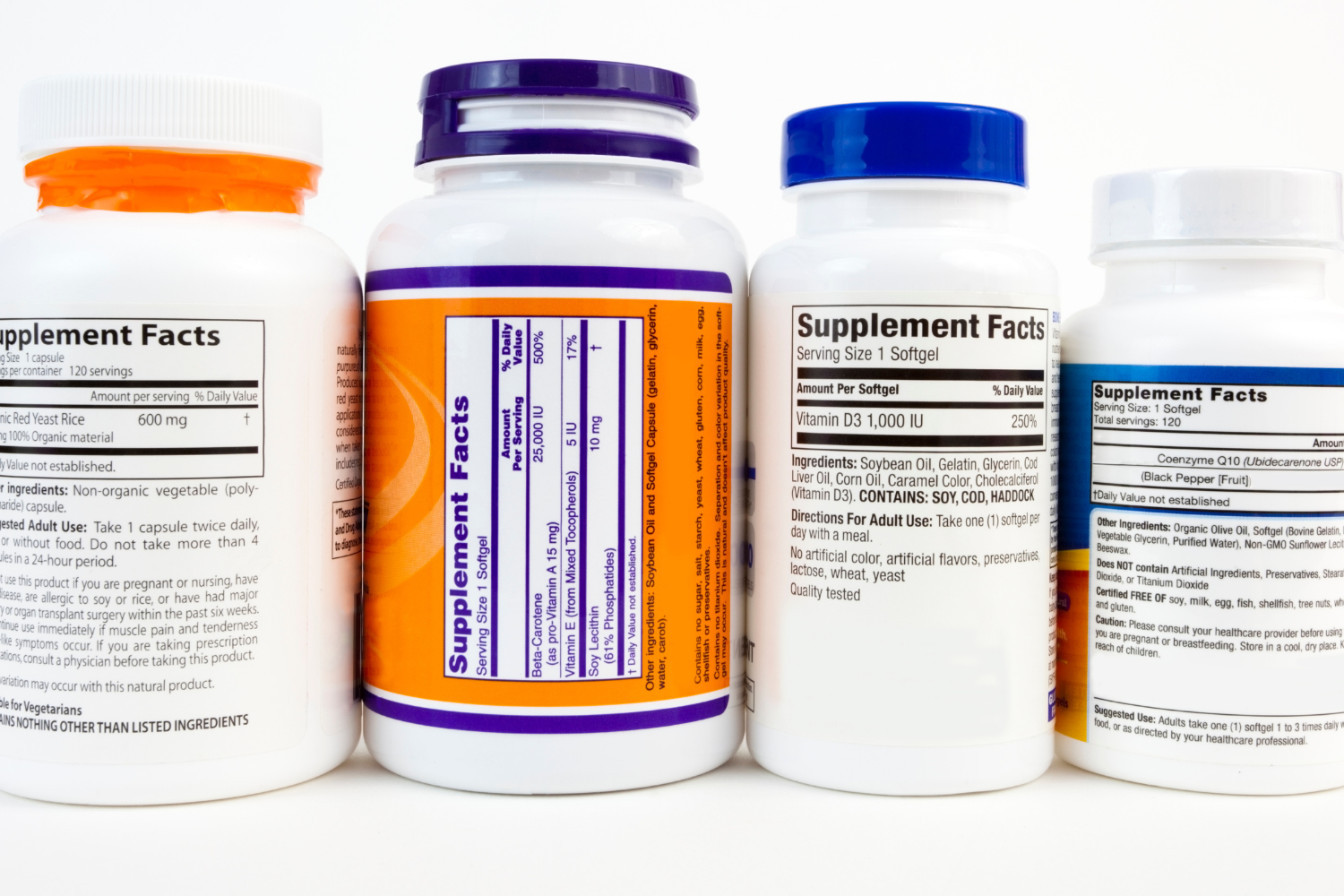
Ensuring Label Accuracy and Clarity
Accuracy is crucial when listing nutrient quantities and daily values. Before printing your supplement labels, double-check all nutrient amounts, such as vitamins, minerals, and amino acids, to ensure they meet the FDA’s recommended daily intake values. This step is essential to avoid mislabeling, which could lead to legal issues or customer distrust.
Making Labels Clear and Legible
Use contrasting colors and clear fonts to ensure your label is easy to read. The text should stand out against the background to improve readability, especially for important information like ingredient lists and %DV. Larger font sizes for headings and bold text for key points will also help consumers quickly find the necessary information.
Special Label Considerations for Different Supplements
Certain types of supplements require special labeling considerations to ensure accurate and compliant information is displayed.
Vitamins and Multivitamins
When labeling multivitamin products, it’s important to list each vitamin and mineral separately, along with their daily values. If your product contains multiple forms of a nutrient (e.g., different types of vitamin D), specify the exact form used. This helps customers understand the precise benefits they are receiving from your multivitamin formula.
Herbal Supplements
Herbal supplements have unique labeling requirements, especially regarding botanical ingredients.
- List the scientific name (Latin name) of each herb.
- Include which part of the plant is used, such as root, leaf, or extract.
- Provide the amount of each herb or extract and any standardization information (e.g., 5% curcuminoids).
Protein and Amino Acid Supplements
For protein and amino acid supplements, you must display the total protein content per serving and, if applicable, the amounts of individual amino acids. This is especially important for athletes and fitness enthusiasts who track their protein intake. Be sure to list these amounts in a way that complies with FDA guidelines and is easy for consumers to understand.

How to Create Custom Supplement Facts Labels
Creating custom supplement facts labels is essential for any dietary supplement business. Whether you design your own or hire a professional, your label must be accurate, compliant, and visually appealing.
Choosing a Label Design Service
Designing your own label or hiring a professional comes with its own set of pros and cons.
Pros of hiring a professional:
- Expert knowledge of FDA regulations
- High-quality, attractive designs that enhance branding
- Saves time, allowing you to focus on other areas of your business
Cons of hiring a professional:
- More expensive than designing it yourself
- Less creative control over the final design
- May involve a longer turnaround time
Printing and Proofing
Ensuring your supplement label is accurate before printing is critical. Follow these steps to proof your label:
- Double-check nutrient quantities and daily values (%DV) for accuracy.
- Ensure compliance with FDA regulations for supplement facts panels.
- Review ingredient lists for any missing dietary ingredients or fillers.
- Confirm that all marketing claims are substantiated and clear.
- Check for typos or formatting issues that might affect readability.
Choosing the Right Printing Material
The right printing material ensures your labels last. Select durable materials that withstand exposure to moisture, temperature changes, and regular handling. Waterproof labels or materials like polypropylene can help keep your product looking professional and intact.
Examples of Supplement Labels Done Right
Here are two examples of how top supplement brands have designed effective and compliant labels.
Case Study 1: A Successful Multivitamin Label
Centrum® Multivitamin is a well-known brand that closely follows FDA regulations. Their supplement facts label lists each vitamin and mineral and the daily value percentage (%DV). This transparency ensures consumers know exactly what nutrients they are consuming. The label design also uses bold fonts for essential details like the serving size, helping customers quickly identify how much to take. Centrum’s commitment to clear labeling builds consumer trust, making it one of the most popular multivitamin brands.

Case Study 2: Protein Supplement Label
Optimum Nutrition’s Gold Standard 100% Whey is an excellent example of a sports supplement highlighting critical information for athletes. Its label prominently displays the total protein content per serving (24g) and breaks down the essential amino acids, including branched-chain amino acids (BCAAs), crucial for muscle recovery. This transparency complies with FDA regulations and helps athletes choose products based on their specific protein and amino acid needs.
Importance of a Clear and Compliant Supplement Facts Label
Having a clear and compliant supplement facts label is crucial for the success of your dietary supplement business. Not only does it ensure that your product meets FDA regulations, but it also builds consumer trust by providing transparency about the ingredients and nutrient content. By carefully following FDA guidelines and avoiding common labeling mistakes, you can protect your business from legal issues and make your product stand out. Start creating your supplement facts label today to ensure your product is compliant and appealing to customers.
Frequently Asked Questions
What is required on a supplement facts label?
To comply with FDA regulations, a supplement facts label must include serving size, servings per container, key dietary ingredients, daily values, and a full ingredient list.
How do I calculate the % Daily Value for my supplement?
To calculate the % Daily Value, use FDA reference values based on a 2,000-calorie diet and ensure accurate measurement of each nutrient in your product.
Can I make health claims on my supplement label?
To avoid misleading consumers and potential legal issues, health claims can only be supported by scientific evidence and approved by the FDA.
What is the difference between supplement facts and nutrition facts labels?
Supplement facts labels are used for dietary supplements, listing dietary ingredients and their amounts, while nutrition facts labels are for food products, focusing on calories and macronutrients.
Why is listing inactive ingredients important?
Inactive ingredients, such as fillers and binders, must be listed on your supplement label to provide full transparency and comply with FDA labeling requirements.
References
- National Institutes of Health, Office of Dietary Supplements. (2023). Dietary Supplements: What You Need to Know. https://ods.od.nih.gov/factsheets/WYNTK-Consumer/
- U.S. Department of Agriculture. (n.d.). Food Labeling. Retrieved from https://www.nal.usda.gov/human-nutrition-and-food-safety/food-labeling
- U.S. Food and Drug Administration. (2021). Dietary Supplements. Retrieved from https://www.fda.gov/consumers/consumer-updates/dietary-supplements
- U.S. Food and Drug Administration. (2005). Dietary Supplement Labeling Guide. Retrieved from https://www.fda.gov/food/dietary-supplements-guidance-documents-regulatory-information/dietary-supplement-labeling-guide
- U.S. Food and Drug Administration. (2017). Guidance for Industry: Dietary Supplement Labeling Guide. Retrieved from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-dietary-supplement-labeling-guide
- U.S. Food and Drug Administration. (2024). The Nutrition Facts Label. Retrieved from https://www.fda.gov/food/nutrition-education-resources-materials/nutrition-facts-label





