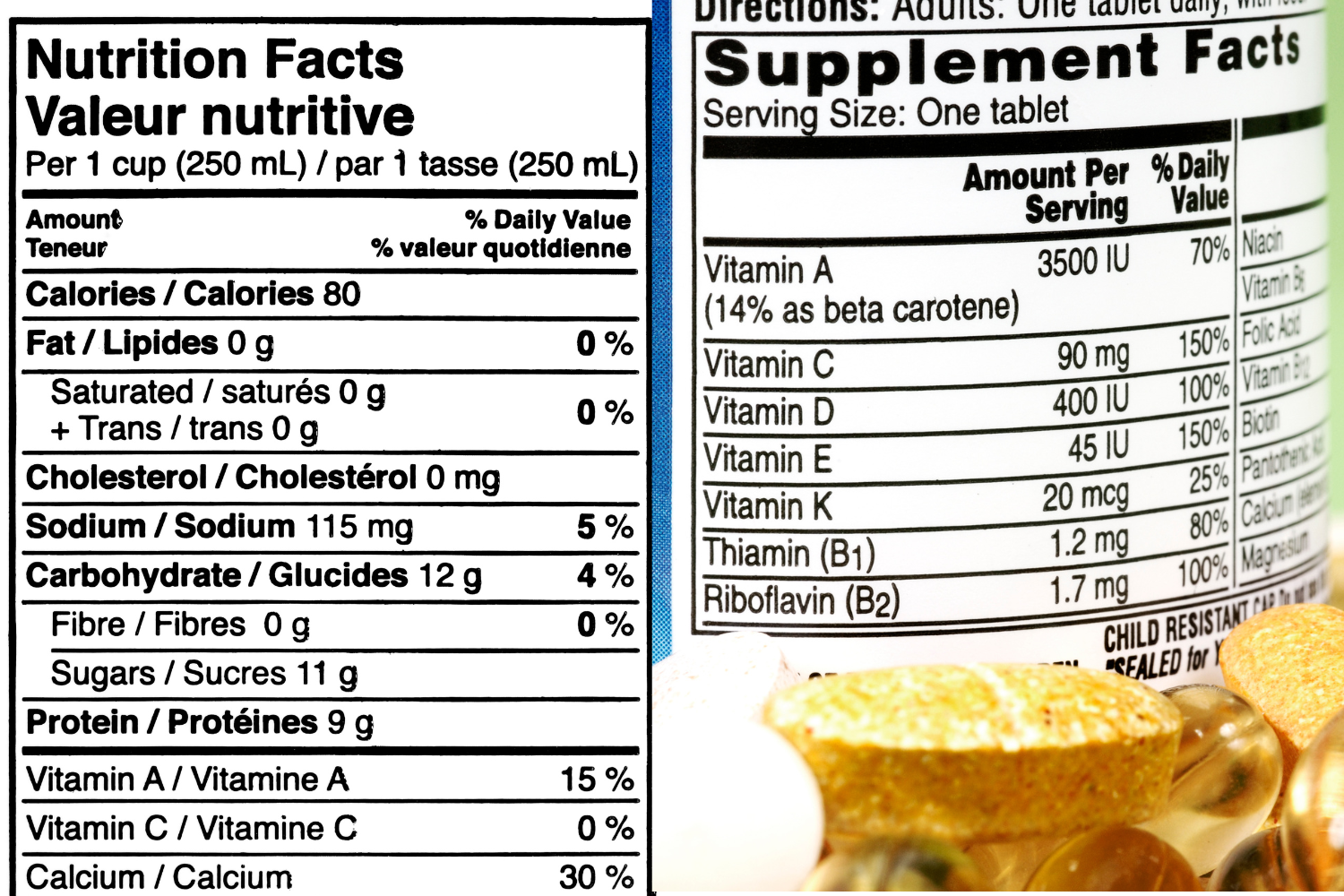
Knowing the difference between Supplement Facts and Nutrition Facts is essential for both consumers and manufacturers when it comes to understanding product labels. These two labels may look similar but serve different purposes and follow distinct regulations.
Whether you’re dealing with dietary supplements or food products, understanding the differences between a supplement facts panel and a nutrition facts panel is key to ensuring proper compliance with FDA regulations and making informed choices. In this guide, we’ll explore the major differences between these labels and how they apply to various products.
What Are Supplement Facts and Nutrition Facts Labels?
Supplement Facts and Nutrition Facts labels are essential tools designed to inform consumers about the contents of dietary supplements and food products. Though both labels provide crucial information, they follow different FDA guidelines and serve various purposes.
Supplement Facts labels are primarily found on dietary supplements, while Nutrition Facts labels are used for packaged food and beverage items. Understanding these differences helps consumers make informed choices and ensures manufacturers comply with legal requirements.
Overview of Supplement Facts Labels
Supplement Facts labels are required for all dietary supplements sold in the U.S. These labels provide detailed information about the dietary ingredients in a product, including vitamins, minerals, and other active components. The FDA requires these labels to help consumers understand what they’re taking and ensure that supplement manufacturers provide accurate nutrient content claims. Supplement Facts labels must include:
- Serving size.
- The amount of each ingredient.
- The % Daily Value (%DV) where applicable.
Products That Use Supplement Facts
Dietary supplements that use Supplement Facts labels include a wide range of products. Here are a few examples:
- Vitamins like Vitamin C and Vitamin D supplements
- Minerals such as calcium, iron, and potassium tablets
- Herbal supplements including echinacea, ginseng, and turmeric

Overview of Nutrition Facts Labels
Nutrition Facts labels are required for all packaged food products and beverages. These labels provide a clear breakdown of a product’s nutritional content, helping consumers manage their diets. The information listed includes details on total calories, macronutrients (like fat, protein, and carbohydrates), and micronutrients. The FDA mandates Nutrition Facts labels to help people make healthier food choices by showing nutrient content and daily value percentages based on a 2,000-calorie diet.
Products That Use Nutrition Facts
Nutrition Facts labels are common on a variety of food and beverage products. Here are some examples:
- Packaged food such as cereal, snacks, and canned goods
- Beverages like soda, juice, and sports drinks
- Protein powders that are used in meal replacements or smoothies

Key Differences Between Supplement Facts and Nutrition Facts
While both labels serve to inform consumers, they follow different FDA guidelines and report different types of information. Understanding these differences is crucial for manufacturers and consumers alike.
FDA Requirements for Each Label
The FDA regulates both Supplement Facts and Nutrition Facts labels, but the requirements for each are unique to the type of product being sold.
What the FDA Requires on Supplement Facts
The FDA requires a Supplement Facts panel with specific details for supplements. These include the serving size, the list of dietary ingredients, the amount of each ingredient, and the % Daily Value (%DV) for vitamins, minerals, and other nutrients. If the product contains a proprietary blend, the total amount of the blend must be listed, but the individual quantities of each ingredient can remain undisclosed.
What the FDA Requires on Nutrition Facts
The FDA mandates a Nutrition Facts label for food products that includes essential details like total calories, total fat, saturated fat, trans fat, total carbohydrates, and total sugars, including added sugars. It lists vital micronutrients such as vitamin D, calcium, iron, and potassium and their corresponding Daily Values (DVs).

Nutritional Content Reporting
Nutritional content is reported differently on Supplement Facts and Nutrition Facts labels, reflecting the differences between dietary supplements and food products.
How Nutrients Are Reported on Supplement Facts
Supplement Facts labels focus on the nutrients and active ingredients specific to dietary supplements. Here are some critical items reported:
- Vitamins like vitamin C or vitamin D support overall health.
- Minerals, such as calcium and magnesium, are vital for bone health and other functions.
- Proprietary blends, which may include herbal ingredients or amino acids.
How Nutrients Are Reported on Nutrition Facts
Nutrition Facts labels on food products report on macronutrients like protein, carbohydrates, and fats. They also include important micronutrients, such as calcium, iron, and vitamin D, to help consumers understand the nutritional value of their food.

Serving Size and Format Differences
Serving sizes are displayed differently on Supplement Facts and Nutrition Facts labels, reflecting the unique nature of supplements and food.
Differences in How Serving Size is Displayed
For supplements, the serving size on Supplement Facts labels typically refers to the amount recommended per serving (e.g., one capsule or one scoop). In contrast, serving sizes on Nutrition Facts labels for food are based on standardized measurements, such as cups, grams, or ounces, depending on the type of food.
Measurement Units Used on Each Label
Supplement Facts labels often list nutrients in smaller units like milligrams (mg), micrograms (mcg), or International Units (IU). On the other hand, Nutrition Facts labels use larger units like grams (g) for nutrients such as total fat, total carbohydrates, and protein, making it easier to understand the nutrient content of foods.
Supplement Facts Label: Detailed Breakdown
Serving Size and Servings Per Container
Choosing the right serving size for supplements is crucial for consumers and manufacturers. The serving size should represent the typical amount a person would consume in one sitting, such as one capsule, tablet, or scoop. This information and the total servings per container must be displayed on the Supplement Facts panel. Manufacturers must ensure this data is accurate to avoid misleading claims and ensure compliance with FDA regulations.
Active Ingredients and Percent Daily Value
When listing active ingredients like vitamins, minerals, amino acids, or herbal extracts on Supplement Facts labels, it’s crucial to display each ingredient’s exact amount per serving. For example, if a supplement contains vitamin C, the amount must be listed in milligrams (mg). The label must also identify other dietary ingredients, such as herbs or specific amino acids like L-glutamine, giving consumers a clear idea of the supplement’s contents.

Calculating Percent Daily Value
The % Daily Value (DV) helps consumers understand how a supplement fits their daily nutritional needs. To calculate the %DV, supplement manufacturers use guidelines set by the FDA based on recommended daily intakes for each nutrient. For example, if a serving of a multivitamin contains 500 mg of vitamin C, and the recommended daily intake is 60 mg, the %DV would be listed as over 800%. This ensures transparency in nutrient content claims.
Other Required Elements
While not all supplements require it, some products may need to list additional information like calories, total fat, or sugar content, especially if these nutrients are present in measurable amounts. For example, a protein shake supplement may include details on total carbohydrates, dietary fiber, and added sugars to provide a more comprehensive understanding of its nutritional content.
Labeling Inactive Ingredients
Inactive ingredients must also be listed on the supplement label, as they play a role in the product’s makeup, though they don’t provide direct nutritional benefits. Here are some common inactive ingredients:
- Fillers, which bulk up the supplement (e.g., cellulose)
- Binders, which help hold the ingredients together (e.g., gelatin)
- Preservatives, which help extend the shelf life of the product (e.g., citric acid)
Nutrition Facts Label: Detailed Breakdown
Calories and Macronutrients
On Nutrition Facts panels, calories represent the total energy provided by a serving of the food. The FDA requires that total calories be prominently displayed near the top of the label, making it easy for consumers to assess the energy content of the product. Manufacturers calculate calories based on the amount of fat, protein, and carbohydrates in each serving. This ensures consumers can make informed decisions about their daily energy intake.
Reporting Fat, Protein, and Carbohydrates
Listing macronutrients on Nutrition Facts labels is essential for helping consumers balance their diets. The label must provide details on the following:
- Total fat, including subcategories like saturated fat and trans fat.
- Total carbohydrates, including subcategories like dietary fiber and added sugars.
- Protein, which helps consumers manage their intake for muscle growth and energy needs.
Key Micronutrients
In addition to macronutrients, Nutrition Facts labels are required to list important micronutrients such as Vitamin D, calcium, iron, and potassium. These nutrients are critical to various bodily functions, including bone health and muscle function. Each micronutrient should include its % Daily Value, helping consumers understand how the food contributes to their recommended daily intake of essential vitamins and minerals.
Additional Information
Manufacturers must include sodium and cholesterol information on food product labels to help consumers manage their intake of these nutrients, which are closely linked to heart health. The FDA requires sodium and cholesterol content to be listed in milligrams (mg) per serving, along with their % Daily Value, to help consumers track their dietary limits.
Ingredient List and Allergen Statements
Food manufacturers need to list all ingredients in order of weight, starting with the heaviest. The FDA also requires food products to identify allergens that may pose a risk to consumers. Here’s what manufacturers should include:
- A complete list of ingredients by weight
- Clear labeling of major allergens, such as peanuts, milk, or soy
- Statements like “may contain” for potential cross-contamination
- The use of common names for allergens (e.g., “wheat” instead of “gluten”)
- Allergen warnings in bold or a separate statement for easy identification
When to Use Supplement Facts vs. Nutrition Facts
Determining Which Label You Need
To determine whether your product requires a Supplement Facts or Nutrition Facts label, it’s important to know how it is classified under FDA regulations. A product is classified as a dietary supplement if it contains dietary ingredients like vitamins, minerals, herbs, or amino acids and is intended to supplement the diet. These products are typically in forms like tablets, capsules, or powders. On the other hand, a product is considered a food product if consumed primarily for taste, aroma, or nutritional value. It provides calories, macronutrients, and other nutrients needed for general nutrition.
Examples of Products Requiring Each Label
Some products are classified under one category or the other. For instance, multivitamins and herbal supplements like echinacea require Supplement Facts labels because they are designed to provide specific nutrients that support the diet. On the other hand, protein bars or ready-to-eat meals require Nutrition Facts labels because they are classified as food products, offering calories and general nutrition. Examples include fish oil supplements (Supplement Facts) versus meal replacement shakes (Nutrition Facts).
Blended Products: When Both Labels Might Apply
Some products combine elements of both dietary supplements and food products, making it tricky to determine the proper labeling. Meal replacement powders, for example, often include essential macronutrients like protein, fat, and carbohydrates, as well as added dietary supplements like vitamins or amino acids. Similarly, fortified snacks like protein cookies or bars may include added nutrients typically found in supplements, such as calcium or vitamin D. In these cases, it’s vital to review the primary purpose of the product—whether it functions mainly as food or a supplement—to decide which label to use.
Dual-Labeling: When You Might Need Both
In some cases, products might require Supplement Facts and Nutrition Facts labels. This dual-labeling applies when a product is marketed as a food and a dietary supplement. For instance, a fortified protein bar with added vitamins or a snack designed to replace a meal may need both labels to comply with FDA labeling guidelines. These products must meet the standards for both categories to ensure consumers receive all relevant information.
Compliance with FDA Labeling Guidelines
Label Layout and Formatting Requirements
The FDA has strict guidelines on how Supplement Facts labels should be formatted and displayed on packaging to ensure clarity for consumers. The Supplement Facts panel must follow specific rules to prevent confusion. Here are some of the key requirements:
- Position: The label must be placed on the information panel, typically on the side or back of the package.
- Font size: The text must be legible, using a font size no smaller than 6-point type.
- Heading: The term “Supplement Facts” must appear at the top of the panel in bold.
- Nutrients: Each dietary ingredient must be listed in order of quantity.
- % Daily Value: This column must be included for each nutrient, if applicable.

Placement and Formatting Rules for Nutrition Facts
Like Supplement Facts, the FDA has clear guidelines for Nutrition Facts labels on food products. Here are some of the requirements for proper formatting:
- Location: The label must appear on the back or side panel of the package in the same field of view as the ingredients list.
- Font size: The font should be at least 8-point type for easy readability.
- Content: The label must include total calories, macronutrients, and micronutrients.
- Bold text: Nutrient names like “Total Fat” or “Carbohydrates” must be bolded.
- % Daily Value: This column must be placed next to each nutrient, showing how the food contributes to daily intake.
Avoiding Common Labeling Violations
To avoid common labeling violations, manufacturers must ensure accurate information on Supplement Facts and Nutrition Facts panels. This includes verifying serving sizes, correctly listing all active and inactive ingredients, and correctly calculating the percent Daily Values. Double-checking label claims, such as nutrient content or health claims, can help prevent misleading information and keep the product compliant with FDA regulations.
Making Health Claims on Labels
Supplement Facts and Nutrition Facts labels may include health claims, but these must be specific and backed by scientific evidence. The FDA allows certain structure/function claims but prohibits misleading statements. Here are some guidelines for making health claims:
- Nutrient content claims: This can only be made if the product meets FDA-defined criteria (e.g., “high in Vitamin C”).
- Health claims: Must be based on established scientific data (e.g., “calcium may reduce the risk of osteoporosis”).
- Structure/function claims: Can state how a nutrient supports normal body function (e.g., “Vitamin D supports immune health”).
- Avoid misleading claims: Do not overstate the health benefits of your product.
- Consult the FDA: Ensure all claims meet FDA standards to avoid fines or product recalls.
Understanding Supplement Facts vs. Nutrition Facts Labels
Supplement Facts labels are required for dietary supplements, which contain specific active ingredients like vitamins, minerals, or herbs, while Nutrition Facts labels are used for food products that provide calories and general nutrition. The key differences between these labels lie in the type of products they apply to and the FDA regulations governing them.
To ensure your product remains compliant and transparent to consumers, it’s important to determine whether it requires a Supplement Facts panel or a Nutrition Facts panel. Start creating the correct label for your product today to meet regulatory standards and build customer trust.
Frequently Asked Questions
What is the difference between Supplement Facts and Nutrition Facts labels?
Supplement Facts labels are used for dietary supplements, while Nutrition Facts labels are required for food products.
How do I know if my product needs a Supplement Facts label?
If your product is a dietary supplement with vitamins, minerals, or other active ingredients, it needs a Supplement Facts label.
Can a product have both a Supplement Facts and a Nutrition Facts label?
Yes, some products, like meal replacement shakes, may require both labels to comply with FDA guidelines.
What information must be included on a Supplement Facts label?
A Supplement Facts label must include serving size, the amount of each dietary ingredient, and the percentage daily value where applicable.
What does a Nutrition Facts label need to show?
A Nutrition Facts label must display total calories, fat, protein, carbohydrates, and critical micronutrients like vitamin D, calcium, and iron.
References
- Food and Drug Administration. (2024). Daily Value on the Nutrition and Supplement Facts Labels. Retrieved from https://www.fda.gov/food/nutrition-facts-label/daily-value-nutrition-and-supplement-facts-labels
- National Institutes of Health, National Center for Complementary and Integrative Health. (n.d.). Dietary and Herbal Supplements | NCCIH. Retrieved fromhttps://www.nccih.nih.gov/health/dietary-and-herbal-supplements
- National Institutes of Health, Office of Dietary Supplements. (n.d.). Frequently Asked Questions (FAQ). Retrived from https://ods.od.nih.gov/Healthinformation/ODS_Frequently_Asked_Questions.aspx
- National Institutes of Health, Office of Dietary Supplements. (n.d.). Dietary Supplement Fact Sheets. Retrieved from https://ods.od.nih.gov/factsheets/list-all/
- U.S. Food and Drug Administration. (2020). Small Entity Compliance Guide: Revision of the Nutrition and Supplement Facts Labels. Retrieved from https://www.fda.gov/regulatory-information/search-fda-guidance-documents/small-entity-compliance-guide-revision-nutrition-and-supplement-facts-labels
- U.S. Food and Drug Administration. (2024). The Nutrition Facts Label. Retrieved from https://www.fda.gov/food/nutrition-education-resources-materials/nutrition-facts-label